The Qdenga™ vaccine is administered as a 0.5 ml dose at a two-dose (0 and 3 months) schedule1
Dose 1
0.5-mL
Administer at Month 0 (Day 1)1
Dose 2
0.5-mL
Administer at Month 3 (Day 91)1
Exploratory data suggests that protection starts building from 3 weeks post first vaccination and persists up to 4.5 years following the second dose1
Help protect your travellers at risk by vaccinating them in time for their travel

- QdengaTM should be administered as a 0.5 mL dose at a two-dose (0 and 3 months) schedule1
- QdengaTM may provide protection from 3 weeks after the first dose is administered, with the second dose completing their vaccination schedule1
QdengaTM is indicated for the prevention of dengue disease (caused by any dengue virus serotype) in individuals from 4 years of age.1
Store QdengaTM between 2-8°C prior to use; do not freeze.1
*The use of QdengaTM should be in accordance with official recommendations.



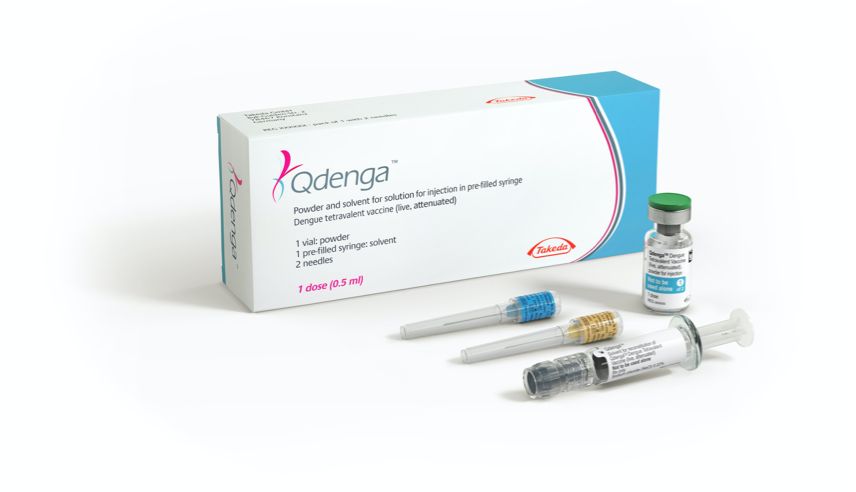
- QdengaTM is available as a powder and solvent for solution for injection, in a pre-filled syringe in the UK and Ireland (not all pack sizes may be marketed in all locations) 1
-
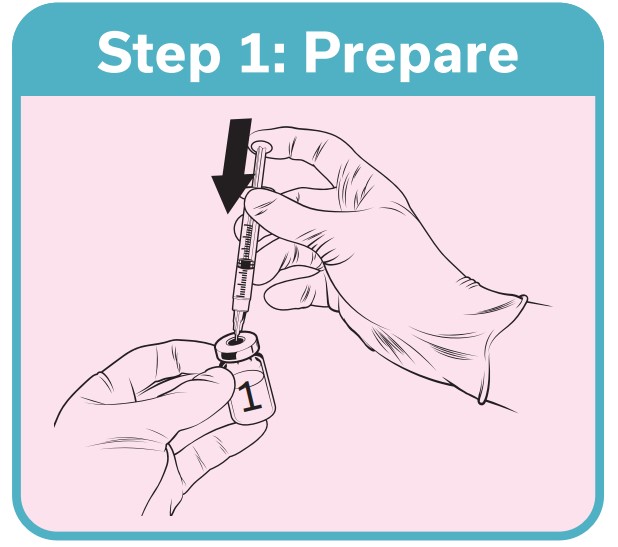
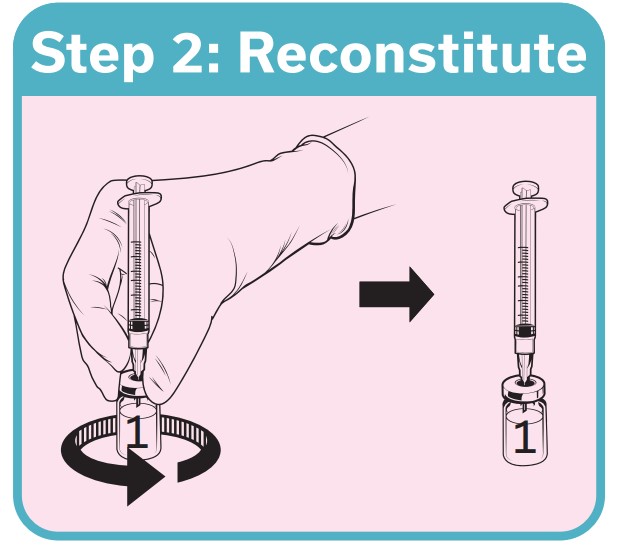
QdengaTM should be reconstituted with the solvent provided prior to administration and used immediately 1
-
If not used immediately, QdengaTM must be used within 2 hours or be discarded 1
-
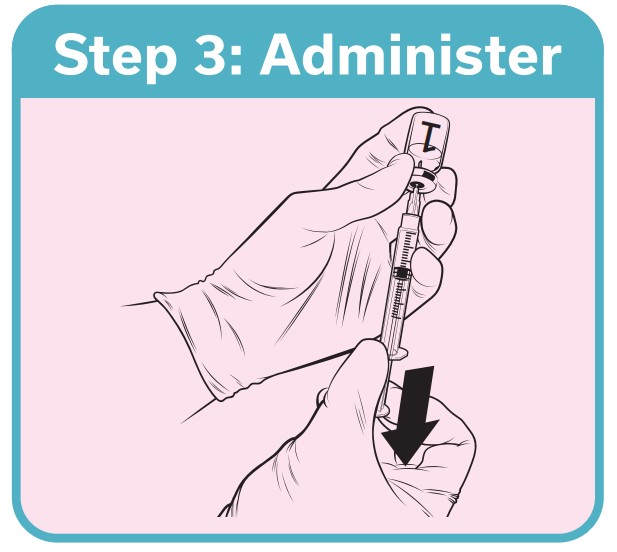
QdengaTM should be administered by subcutaneous injection, preferably in the upper arm in the region of deltoid
-
QdengaTM must not be administered by intravascular, intradermal or intramuscular injection
-
The vaccine should not be mixed in the same syringe with any vaccines or other parenteral medicinal products1 (please see section 6.6 of SmPC for instructions on reconstitution of QdengaTM before administration)
-
The need for a booster dose has not been established 1
-
As with all injectable vaccines, appropriate medical treatment and supervision must always be readily available in the event of a rare anaphylactic reaction following administration of the vaccine.
Development
Takeda has more than 70 years' experience in developing and manufacturing vaccines in Japan
QdengaTM is developed and prepared under cGMP conditions in accordance with the latest regulatory requirements:2-3
DENV-2 virus was isolated from nature and attenuated. Later, using recombinant techniques, the other chimeric viruses DENV-1, DENV-3 and DENV-4 were developed using the attenuated DENV-2 virus 4
The DENV-2 strain is attenuated and, using recombination techniques, the other 3 chimeric viruses are formed by replacing the prM and E protein genes from DENV-1, -3 and -4 within the DENV-2 backbone 3
Other non-active ingredients, including stabilisers, are added to allow for a viable formulation 3
The quality and potency (i.e. biological activity) of each live, attenuated, tetravalent formulation is checked to help ensure the quality, integrity and security of every product
▼ This medicinal product is subject to additional monitoring.
GB & NI: Adverse Events should be reported. Reporting forms and information can be found at: www.mhra.gov.uk/yellowcard.
ROI: Adverse Events should be reported to the Pharmacovigilance Unit at the Health Products Regulatory Authority. Reporting forms and information can be found at: www.hpra.ie.
GB, NI and ROI: Adverse Events should also be reported to Takeda UK Ltd at: AE.GBR-IRL@takeda.com
- QdengaTM Summary of Product Characteristics.
- Takeda Newsroom. Takeda unveils new dengue vaccine manufacturing plant in Germany. November 05,2019. https://www.takeda.com/newsroom/newsreleases/2019/takeda-unveils-new-dengue-vaccine-manufacturing-plant-in-germany/. Retrieved October 2023. Last accessed October, 2025.
- Osorio JE, et al. Vaccine. 2015;33(50):7112-7120.
- Huang CY-H, et al. PLoS Negl Trop Dis. 2013;7(5):e2243.
AE, adverse event; CI, confidence interval; DCAC, dengue case adjudication committee; DENV, dengue virus; DHF, dengue hemorrhagic fever; GMT, geometric mean titer; RT-PCR, reverse transcription polymerase chain reaction; TDV, the DENV [dengue virus] strain; VCD, virologically confirmed dengue; WHO, World Health Organization.
C-APROM/GB/DENV/0212 | Date of preparation: October 2025

